Philips Begins Repair and Replacement of First Generation DreamStation Respiratory Care Devices
Image source: Recall Notification, Philips Respironics Sleep and Respiratory Care devices
Health technology giant Koninklijke Philips N.V (NYSE: PHG ) will soon start repairing and replacing millions of respiratory devices in the US to address potential health risks posed by the machines.
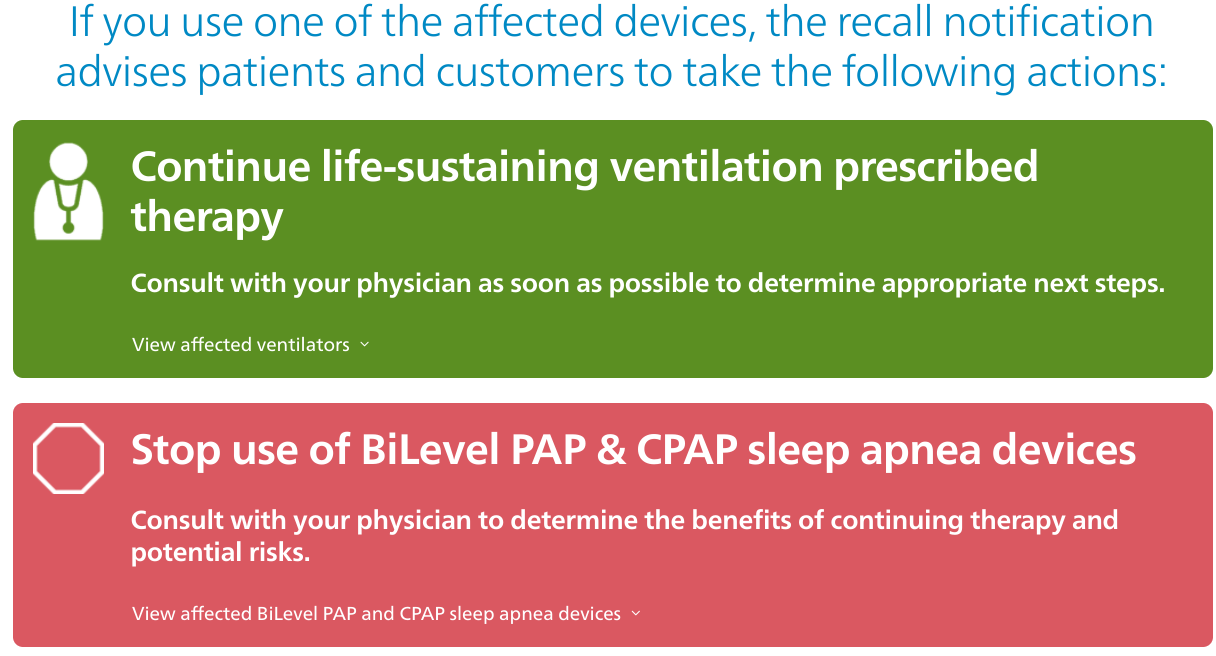
In June, the Dutch company recalled four million of its CPAP, BiPAP and ventilator devices, including its first generation DreamStation machines, after finding that the polyurethane (PE-PUR) sound abatement foam used in these products could degrade, become toxic and potentially cause cancer.
In a press release on Wednesday, Philips said the US Food and Drug Administration (FDA) authorized the rework of the DreamStation devices, which involves replacing the sound abatement foam with a new material.
Eighty percent of the affected machines were sold in the US. The entire replacement effort will take about a year and cost about $601 million.
MedTech Dive noted that the recall was categorized as a “Class I” event by the FDA, the agency’s severest recall labeling. According to the FDA, more than 100 injuries and 1,200 complaints have been reported over the products.
As a result, Philips is facing a growing number of class action lawsuits.
In a statement on Wednesday, Frans van Houten, chief executive officer of Philips, said, “We fully recognize that the timeframe for remediation of the affected devices places patients in a difficult situation.”
“We are mobilized to deliver a solution to them as fast as possible. We have significantly increased our production, service and rework capacity, and further intensified our outreach to our customers and their patients. We urge patients with affected active devices to register these on the dedicated recall notification website.”
_____
Source: Equities News