TG Therapeutics Tumbles 40% on Partial Clinical Trial Hold
 Image source: TG Therapeutics
Image source: TG Therapeutics
Shares of TG Therapeutics (TGTX) got punished Thursday after CEO Michael Weiss disclosed that the FDA had placed a partial clinical hold on certain of the company's Phase 3 studies in chronic lymphocytic leukemia (CLL) and non-Hodgkin's lymphoma (NHL).
The stock closed at $8.27, down 40.5% on the day.
Weiss was speaking on a webcast fireside chat during the B. Riley Securities 2022 Virtual Oncology Investor Conference.
Partial clinical hold
TG Therapeutics received accelerated FDA approval in February 2021 for its oral kinase inhibitor, Ukoniq (umbralisib), in the treatment of relapsed or refractory marginal zone lymphoma (MZL) and relapsed or refractory follicular lymphoma (FL).
The company has been studying Ukoniq in combination with a monoclonal antibody, ublituximab, for the treatment of CLL and NHL.
Weiss said Thursday that, per the partial clinical hold:
- No new patients may be enrolled to the select CLL/NHL studies identified by FDA.
- Patients on these studies who are deriving clinical benefit can elect to continue on therapy.
Is this a buying opportunity?
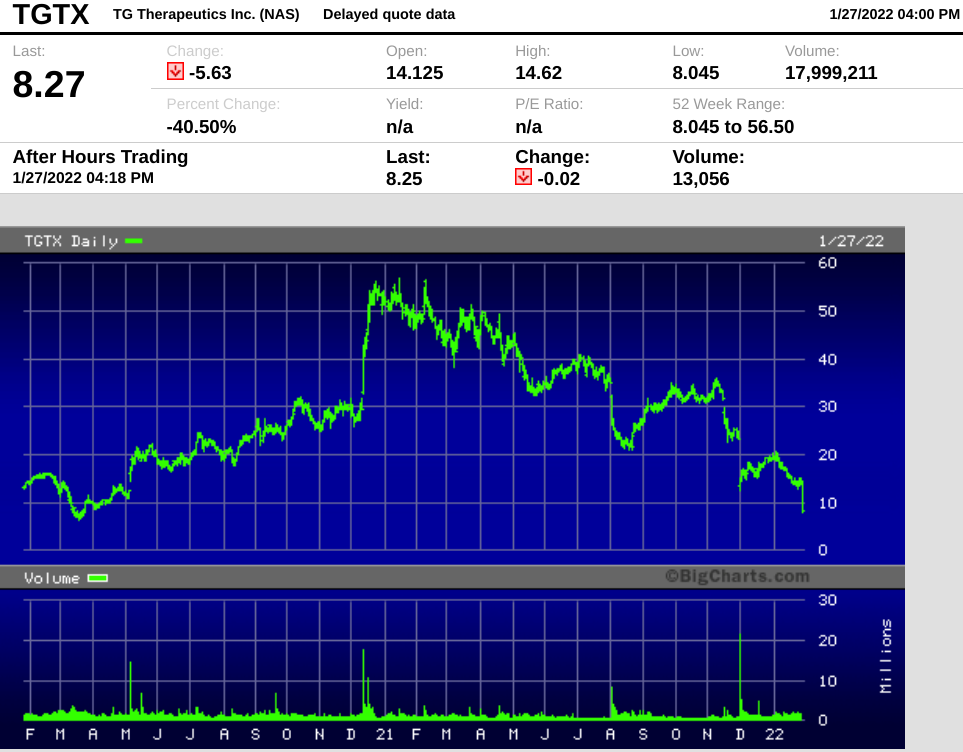
Image source: BigCharts
The stock breached $56 per share in December 2020, in the run-up to Ukoniq's approval. Investors have obviously been disappointed in the time since then, but today's sharp sell-off appears to be an overreaction.
- Weiss stressed that the partial clinical hold was not based on any new information provided by the company to the FDA. Rather, he said that the FDA's decision was based on the same data and concerns that led to the scheduling of an Oncologic Drug Advisory Committee (ODAC) meeting, which the company disclosed on November 30, 2021. That meeting is due to occur in March or April 2022.
- Additionally, Weiss said that the updated overall survival (OS) results from the Phase 3 study showed an improvement from the preliminary data originally shared with the FDA and previously disclosed on November 30, 2021
-
In December 2021, the company presented data from 210 patients with both treatment naïve (119) and relapsed or refractory (91) CLL at the American Society of Hematology annual meeting. The data on the treatment naïve group were particularly compelling:
- Two-year progression-free survival (PFS) was 76.6% with PFS of 38.5 months.
- Overall response rate (ORR) in the TN population was 84%.
TG Therapeutics has multiple investigational drugs in the pipeline, and the approval of Ukoniq last year served to validate the company's scientific approach.
We think the current price represents a value entry point for investors who are willing to accept the high risks and binary outcomes inherent to biotech investing.
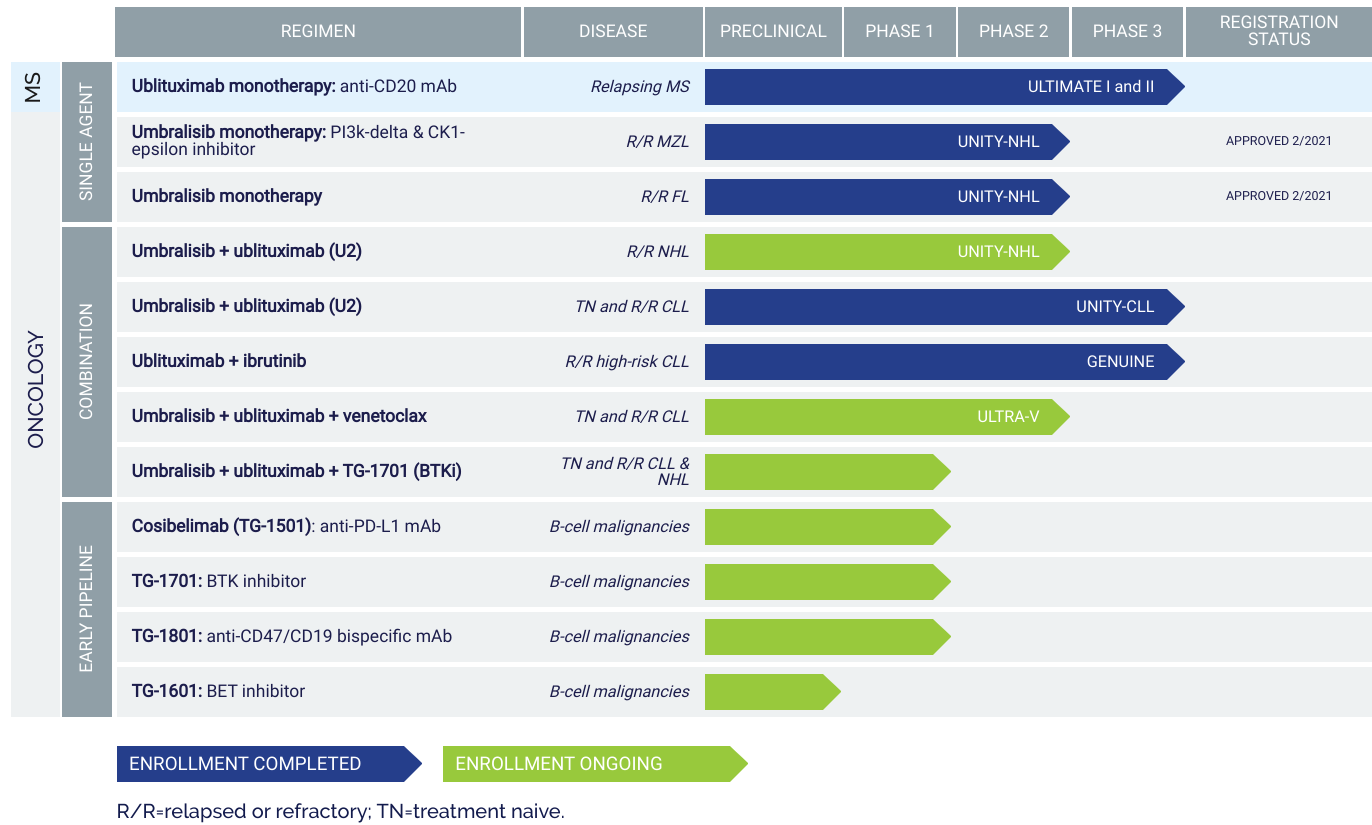
Image source: TG Therapeutics
_____
Source: Equities News



