Sage Therapeutics Gets FDA Breakthrough Therapy Designation for Major Depressive Disorder Drug
Sage Therapeutics (Nasdaq: SAGE) today announced that the FDA granted Breakthrough Therapy designation to one of its lead candidates, SAGE-217, for the treatment of major depressive disorder (MDD). This is the second Breakthrough Therapy designation granted to Sage since 2016.
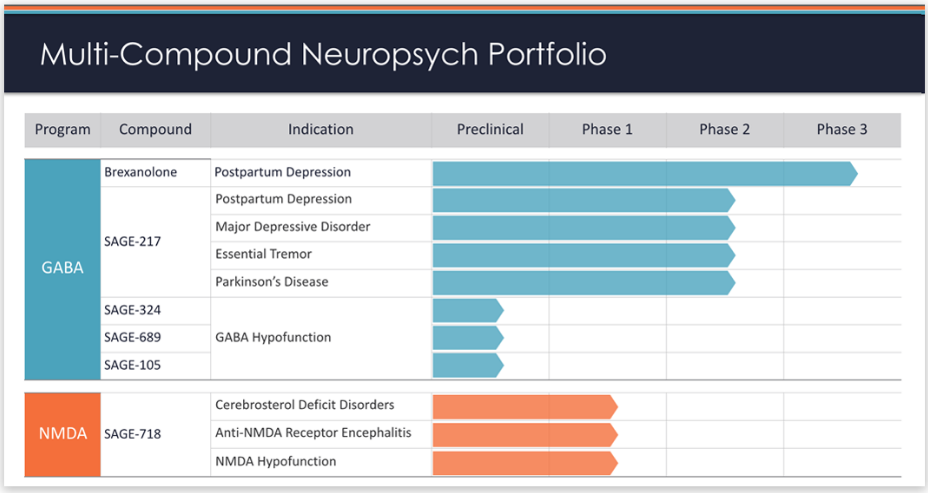
SAGE-217 is an oral drug candidate that targets synaptic and extrasynaptic GABA receptors, part of the major inhibitory signaling pathway of the brain and central nervous system. SAGE-217 is currently being developed in the treatment of MDD and certain other affective disorders, Parkinson’s disease and sleep disorders.
The Breakthrough Therapy designation is intended to offer a potentially expedited development path and review for promising drug candidates, which includes increased interaction and guidance from the FDA. This regulatory decision was based primarily on the recent positive results from the Phase 2, placebo-controlled trial of SAGE-217 in 89 adult patients with moderate to severe MDD. Detailed results of the Phase 2, placebo-controlled trial will be presented at an upcoming medical meeting.
Sage Therapeutics has a pipeline of drug candidates targeting critical CNS receptor systems, GABA and NMDA. Sage’s lead program, a proprietary IV formulation of brexanolone (SAGE-547), has completed two Phase 3 clinical trials in postpartum depression. Sage is developing its next generation modulators, including SAGE-217 and SAGE-718, in various CNS disorders.
Please email us at [email protected] to see our Case Studies and Testimonials.
Please click here if you would like information on our new trading platform.
Please click here if you would like to see our weekly newsletter.