Regulus Therapeutics — Microcap Play in Targeted MicroRNA Medicines
Video source: Regulus Therapeutics
Regulus Therapeutics ( RGLS ) reported fourth quarter and full year 2021 results on Thursday.
Net loss for the quarter was $7.1 million, or $0.07 per share, compared to a loss of $1.3 million, or $0.03 per share, in 2020.
Full year net loss was $27.8 million, or $0.32 per share, compared to a loss of $15.7 million, or $0.45 per share in 2020.
Regulus is discovering and developing medicines targeting microRNAs. Dysregulated microRNA expression is a key factor in many complex diseases, including inflammatory disease, fibrosis, metabolic disease and cancer.
MicroRNA therapeutics are oligonucleotides that modulate the function of microRNAs, correcting the imbalance of gene expression and associated cellular pathways to treat a broad range of human disease.
As with all pre-revenue biotechs, the importance of quarterly and annual results lies in the clinical and fundraising progress made, and to this end, Regulus has had several positive recent developments.
Highlights
Alport Syndrome: Regulus announced in February 2022 the completion of its enrollment for its Phase 2 study evaluating its candidate lademirsen for Alport syndrome under its deal with Sanofi (SNY). Alport syndrome is a disease that attacks the tiny blood vessels in kidneys.
Autosomal Dominant Polycystic Kidney Disease (ADPKD): Regulus completed a pre-IND meeting with the FDA in January 2022 for its candidate RGLS8429. The FDA provided overall agreement with the trial design and length of the Phase 1 study.
Private placement: The company raised $34.6 million in gross proceeds via a private placement led by the Federated Hermes Kaufmann Funds and New Enterprise Associates (NEA), with participation from other new and existing investors.
Investment thesis
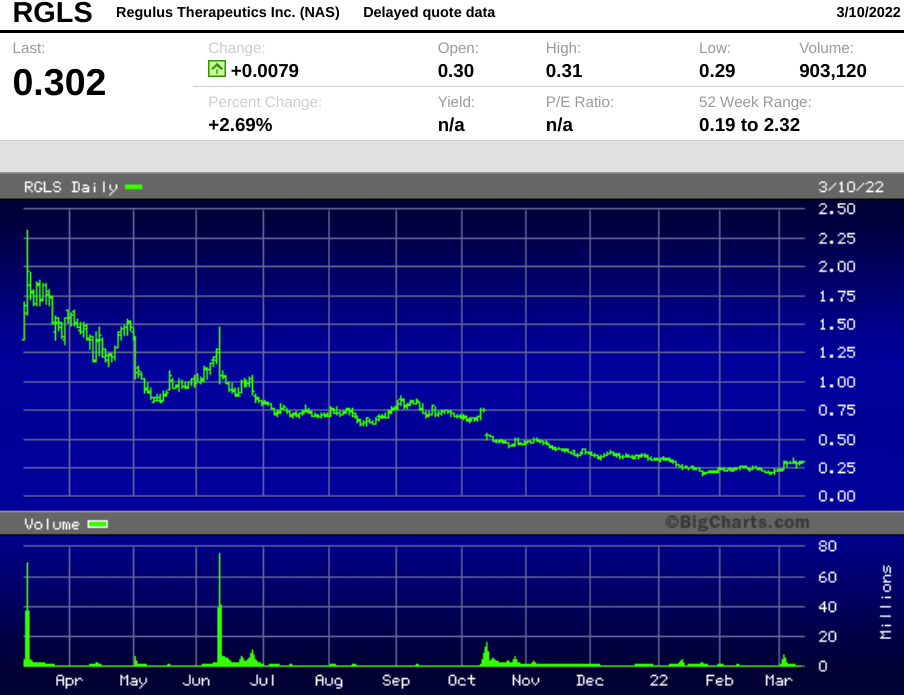
Regulus went public amid some fanfare in 2012, but the stock hasn't given investors much to cheer about for the past several years. At Thursday's close of $0.302, Regulus has a market capitalization of just $44 million.
- The company had $60.4 million in cash and equivalents as of Dec. 31, 2021, so the stock is trading below its cash value. This is obviously not for risk-averse investors, but those who choose to play are getting a free call option on the company's therapeutic portfolio.
- Existing cash gives the company a runway through 2023.
- Final data on the Phase 2 trial of lademirsen for Alport Syndrome is expected in the first half of 2023 and if successful could provide further validation of the company's platform technology designed to address genetic kidney diseases and earn Regulus a $25 million milestone payment from Sanofi, furthering the cash runway into 2024.
- Regulus is on track to submit an IND application in Q2 2022 for its ADPKD treatment. Top-line data would be expected in the second half of 2022, and top-line biomarker data in the first half of 2023.
_____
Source: Equities News



