Impel NeuroPharma Announces $100 Million Royalty and Debt Financing With Oaktree

Image source: Impel NeuroPharma
Impel NeuroPharma (IMPL) announced on Friday two separate transactions with funds managed by Oaktree Capital Management totaling $100 million in gross funding.
The transactions include a $50 million royalty agreement on net sales of Impel's nasal spray for migraines, Trudhesa, and $50 million of senior secured debt.
"This non-dilutive financing provides Impel with immediate and sufficient capital to support the continued successful launch and commercialization efforts for Trudhesa.” said Adrian Adams, Chairman and CEO of Impel
“Additionally, this transaction meets our objectives of strengthening our balance sheet, while retaining for our shareholders the majority of upside from growing Trudhesa sales.”
Oaktree has $166 billion in assets under management.
Under the terms of the royalty agreement, Oaktree will pay Impel an upfront cash payment of $50 million in exchange for tiered royalty payments on annual US net sales of Trudhesa, as follows:
- 7.75% on net sales up to $150 million
- 4.75% on net sales between $150.0 million and $300 million
- 0.75% on net sales greater than $300 million
- 10.0% of any payments received by Impel from ex-U.S. licensing or partnership deals
The total royalty payable by Impel to Oaktree is capped at 1.75x of the amount funded upfront, with the ability to redeem the royalty agreement at lower multiples within the first three years from funding. The royalty agreement will terminate upon achievement of the cap amount,
Under the terms of the credit facility, Impel will borrow $50 million from Oaktree, all of which will be funded at closing. The credit facility will mature in five years, bearing interest at the secured overnight financing rate (SOFR) + 8.75%, with an SOFR floor of 1.00%.
The interest rate margin will be lowered to 8.00% after Impel achieves $125 million in trailing twelve-month U.S. net sales. Impel will make interest-only payments for the first 48 months and will retire its existing $30 million debt obligation with Oxford/SVB.
Precision Olfactory Delivery (POD) technology
Impel offers and is developing treatments that pair its proprietary Precision Olfactory Delivery (POD) technology with well-established therapeutics. In addition to Trudhesa (dihydroergotamine mesylate) nasal spray, which is approved in the US for the acute treatment of migraine with or without aura in adults, Impel is also developing INP105 for the acute treatment of agitation and aggression in patients with autism, and INP107 for OFF episodes in Parkinson’s disease.
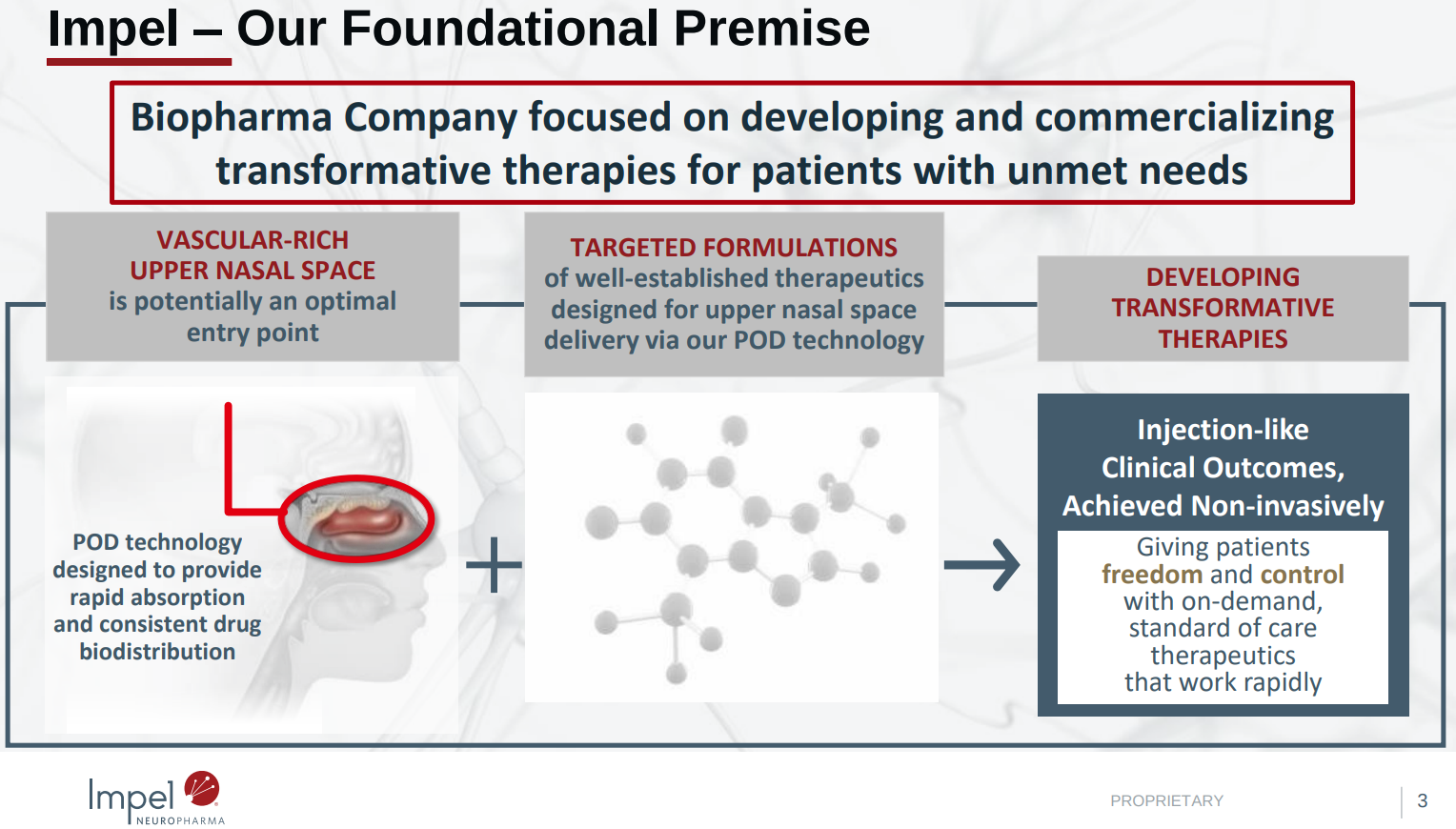 Image source: Impel NeuroPharma
Image source: Impel NeuroPharma

Investment thesis
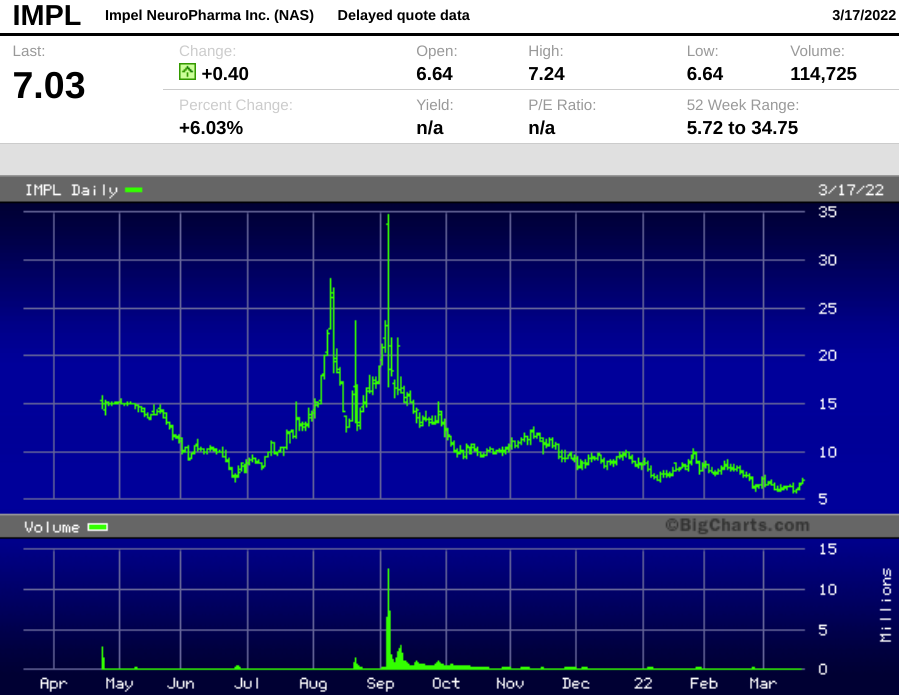
Impel went public in April 2021, raising $80 million in gross proceeds at $15 per share. The company raised an additional $45 million in September 2021 at the same price, which came at a steep discount to the closing price on deal day of $21.13. The stock has drifted lower since then, but we think investors have not fully appreciated the potential of the nasal spray technology.
- It's rare to see a company with an FDA-approved product that treats a major indication trading at such a low valuation.
- Current market cap of $172 million makes the stock akin to a low-priced call option on sales of its migraine nasal spray.
- The company's POD technology has been clinically validated and has potentially very broad utility across a range of indications.
- Market for migraine medications is enormous, with 30 million annual prescriptions.
- The company's INP105 is poised to fill a major unmet need for acute treatment of agitation and aggression in autism
- This latest financing, which is nondilutive, provides the company with a projected cash runway into 2024.
_____
Source: Equities News



