Fusion Pharmaceuticals Closes $75 Million Credit Facility With Oxford Finance
Video source: YouTube, Fusion Pharmaceuticals
Fusion Pharmaceuticals ( FUSN ) has closed a $75 million senior secured term loan by Oxford Finance.
Fusion is developing next-generation radiopharmaceuticals as precision medicines to treat a broad range of cancers in areas of high unmet need. The company connects alpha particle emitting isotopes to various targeting molecules in order to selectively deliver the alpha emitting payloads to tumors.
Oxford has been providing senior secured loans to public and private life sciences and healthcare services companies for over 20 years, originating approximately $8.8 billion in total loans.
Fusion's "TAT [targeted alpha therapy] platform has the potential to enhance the tumor-killing power of radiation and is designed for broader applicability across multiple tumor types,” said Christopher A. Herr, senior managing director at Oxford.
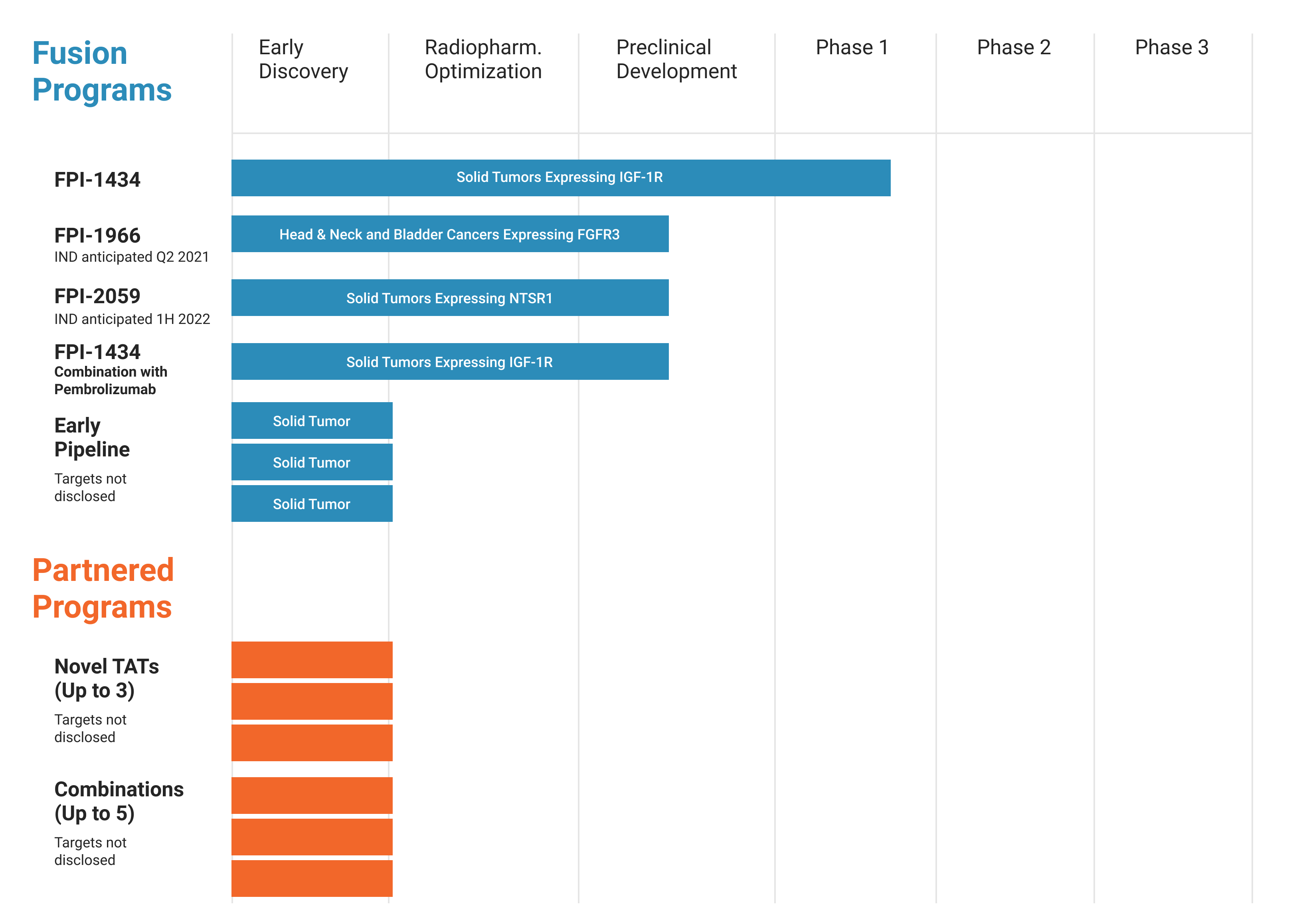 Image source: Fusion Pharmaceuticals
Image source: Fusion Pharmaceuticals
Investment thesis
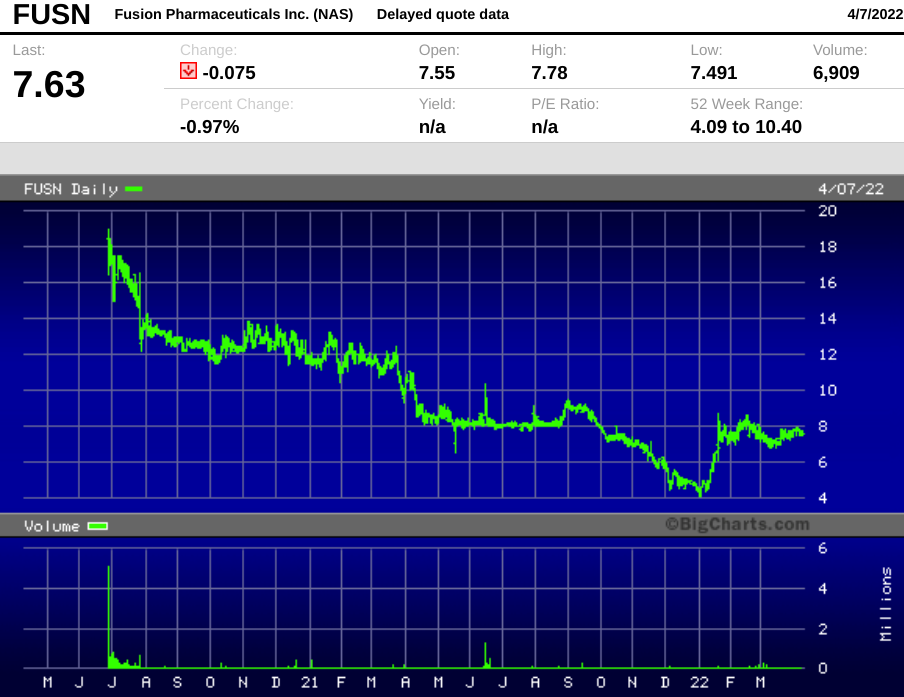
Fusion Pharmaceuticals went public in June 2020, raising $213 million in gross proceeds at $17 per share via Morgan Stanley, Jefferies and Cowen. The post-IPO market cap was $708 million, but the stock fell below issue price after a week and has never regained the level.
The market cap as of Tuesday's close of $7.62 is only $330 million.
While the stock has been a disappointment to date, we think investors have reasons for optimism, especially at current levels.
- As of Dec. 31, 2021, Fusion had cash and equivalents of $221 million, which the company said last month would be enough to fund operations through 2023.
- This latest $75 million credit facility provides a welcome cushion.
- The company has numerous collaborations with premier companies and institutions, including Astra Zeneca, Merck and McMaster University of Ontario.
-
Fusion has several milestone events on the horizon which could be catalysts for the stock.
- The first patients in the Phase 1 trial of FPI-1966 for solid tumors expressing FGFR3 will be dosed in the second quarter of 2022.
- FDA submission of an IND for FPI-2059, Fusion's first small molecule program, is expected in the second quarter of 2022.
- Data from the Phase 1 trial of FPI-1434 in patients with solid tumors is expected in the second half of 2022.
- Radiopharmaceuticals as a class of drugs holds great promise among cancer researchers. Charles Kunos, MD, PhD, of the National Cancer Institute's Cancer Therapy Evaluation Program (CTEP) says radiopharmaceuticals will "transform radiation oncology in the next 10 to 15 years."
_____
Source: Equities News



