Eton Pharmaceuticals — Small Cap Orphan Drug + Royalty Hybrid With Multiple FDA Arrows

Image source: Eton Pharmaceuticals
Eton Pharmaceutics ( ETON ) reported fourth quarter 2021 results on Wednesday and provided an update on its broad portfolio of commercial products and pending FDA applications.
The company reported revenue of $6.1 million for Q4 after reporting no material revenue in Q4 2020. Most of this consisted of a $5.0 million milestone payment from Azurity Pharmaceuticals triggered by the commercial launch of Eprontia for seizures and migraines.
Eton reported net income of $1.0 million for the fourth quarter of 2021, or $0.04 per share, compared to a net loss of $7.7 million in the prior-year period, or a loss of $0.32 per share.
“Our business has never been stronger," said Sean Brynjelsen, CEO of Eton Pharmaceuticals.
"The carglumic acid launch is tracking ahead of our initial expectations, the recently implemented co-promotion partnership with Tolmar has accelerated growth of Alkindi Sprinkle and just this week we launched another new product, Rezipres.”
Differentiated business model
Pursuing multiple development pathways.
Reducing reliance on any single product, therapeutic area, or technology in our portfolio drives our commitment to building a diversified pipeline. We do that by maintaining deep relationships globally that lead to:
Acquisitions
Joint ventures
Partnerships
Product licensing
Investment thesis
Eton has quietly been making notable progress with multiple drug approvals. The company's strategy is to go after relatively easy wins, i.e., drugs that have a high likelihood of approval because they are:
- Proprietary formulations of approved molecules,
- Safer, more effective, or more affordable than existing products
- Treating potential new indications
The current market cap of only $103 million feels like good value for a company with multiple revenue streams and lean operating model.
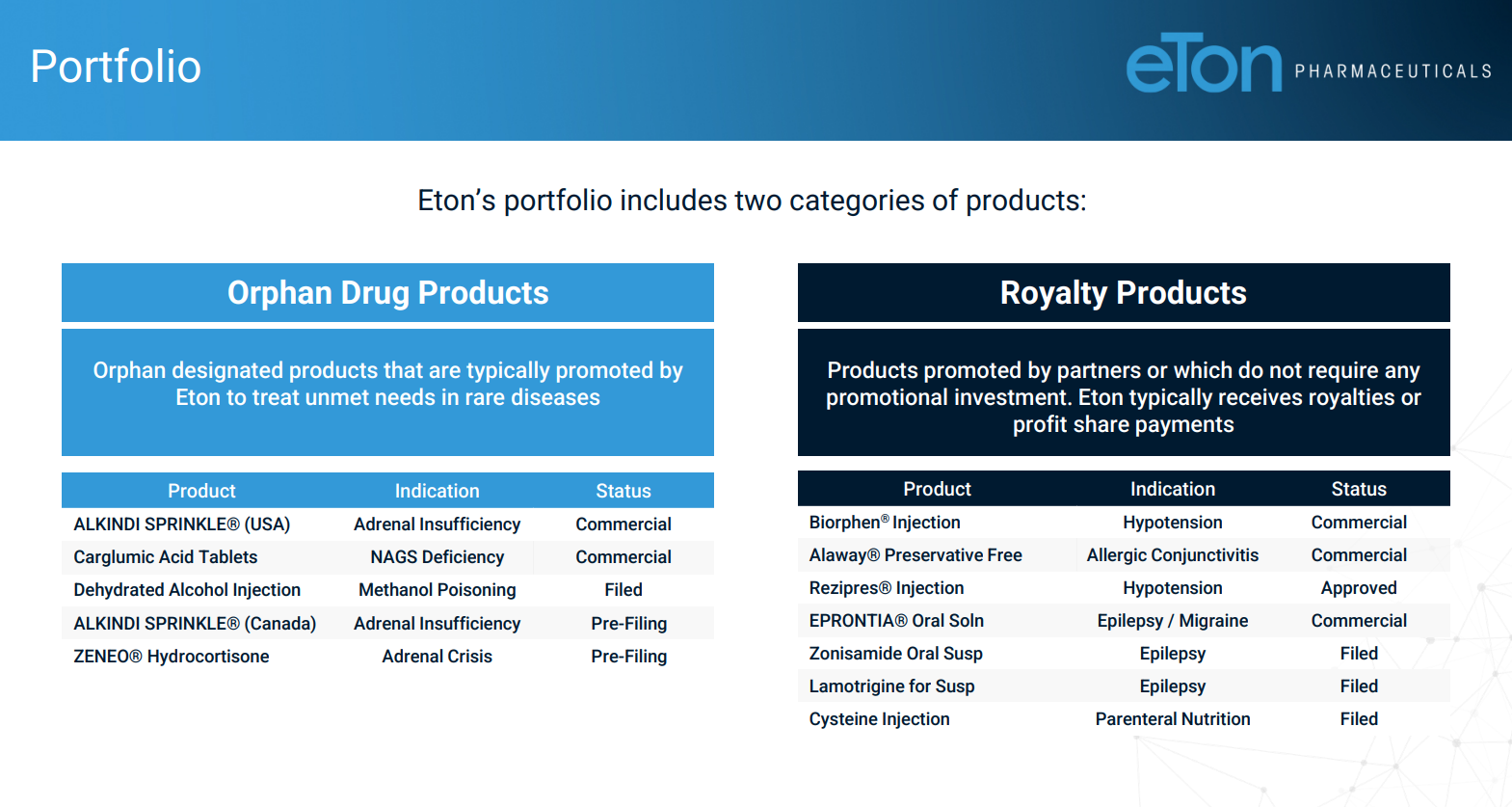
- In addition to owning or receiving royalties from six products, the company has filed submissions for four additional products to the FDA that it believes have a high likelihood of approval in 2022.
- Eton stock took a beating last May when the FDA sent a complete response letter re the company's dehydrated alcohol injection treatment for methanol poisoning. The company stated in its earnings press release that it is "actively working to prepare a resubmission to the FDA that addresses all of the FDA’s questions from the Complete Response Letter and items discussed during its meeting with the FDA that took place in the fourth quarter. Eton is confident that it will be able to fully address all of the FDA’s requests and expects to have the response submitted in the coming weeks."
-
The company targets rare diseases with critical unmet need for therapies, e.g.,
- Eton's carglumic acid is the first generic formulation of Carbaglu, the treatment for blood ammonia whose annual cost exceeds $1 million for many patients.
- Alkindi Sprinkle the first and only FDA-approved granular hydrocortisone formulation for the treatment of adrenocortical insufficiency specifically designed for use in children.
- Rezipres is Eton's injectable ephedrine hydrochloride formulation that is approved for the treatment of clinically important hypotension occurring in the setting of anesthesia.
- The royalty side of the business requires no promotional investment.
- The company's lean operating model should drive continued profitability.
_____
Source: Equities News



