Chinook Therapeutics Raises $105 Million for Kidney Disease Therapies
Chinook Therapeutics ( KDNY ) announced Tuesday that it has priced a follow-on equity offering to raise gross proceeds of $105 million in common stock and pre-funded warrants at $14.00 per share.
The Seattle-based developer of precision medicines for kidney diseases is selling 6,428,572 shares of common stock and pre-funded warrants to purchase up to 1,071,428 shares of common stock.
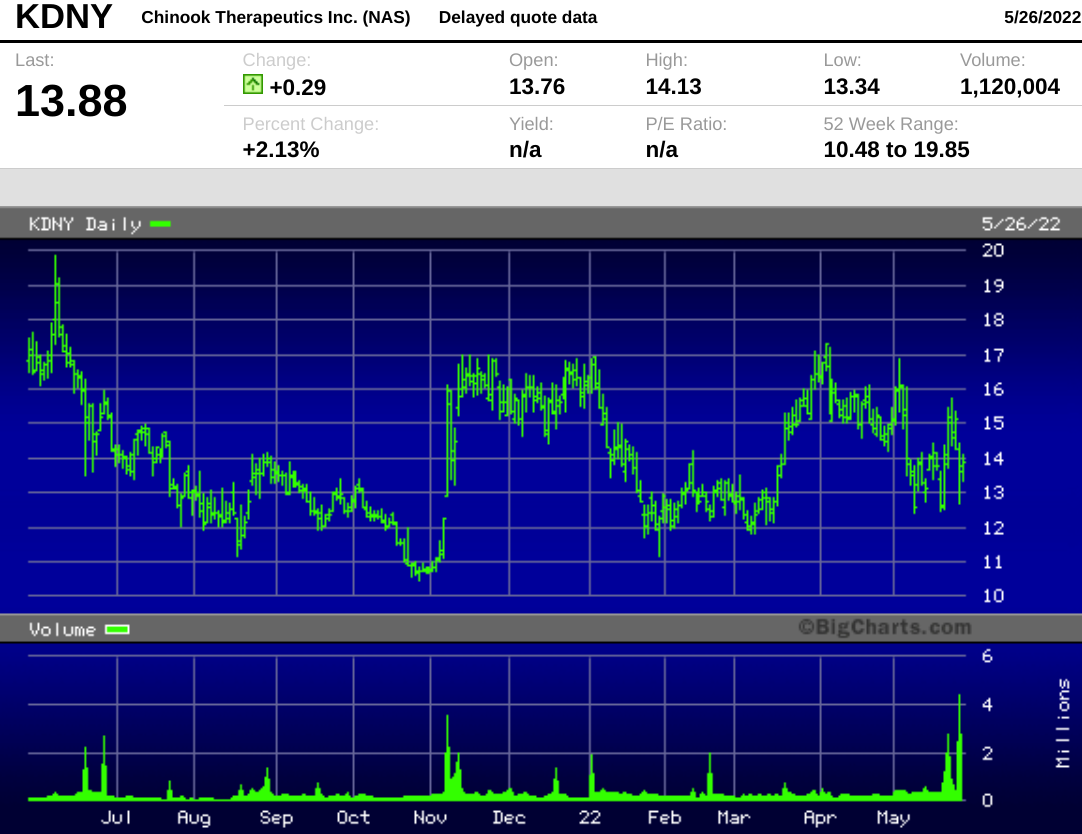
The $14.00 deal price represented a 7.3% discount to Tuesday's closing price of $15.11.
The company took advantage of the increase in stock price in the wake of last week's presentation of positive clinical trial data from their two leading programs at the 59th European Renal Association (ERA) Congress 2022 that was held virtually and live in Paris.
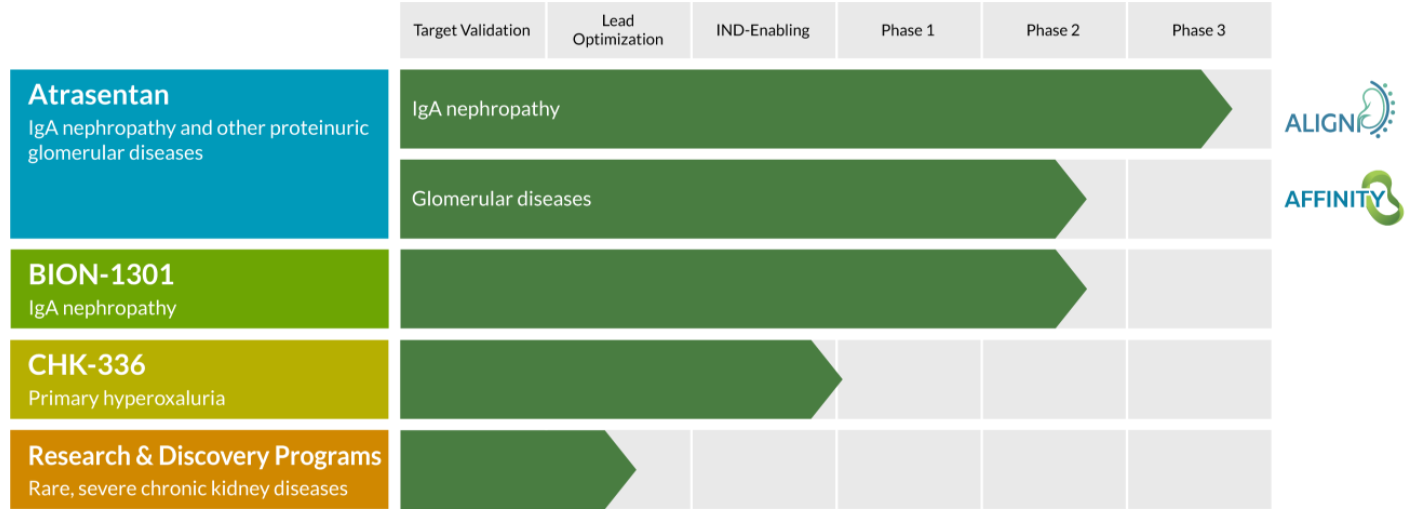
BION-1301, an anti-APRIL (A Proliferation Inducing Ligand) monoclonal antibody in a Phase 1/2 trial for patients with Immunoglobulin A Nephropathy (IgAN), or Berger's disease.
The company presented data showing reductions in biomarkers and in proteinuria (protein levels) that indicate kidney disease.
Atrasentan, an inhibitor of the endothelin A receptor, is in the enrollment stage for a Phase 3 trial for the potential treatment of IgAN.
Chinook presented data showing "highly consistent and clinically meaningful" proteinuria reductions at weeks six, 12 and 24 of treatment in patients with IgAN who are already on a maximally tolerated and stable dose of a RAS inhibitor.
President and CEO Eric Dobmeier said, "This level of proteinuria reduction is likely to translate into significant clinical benefit for patients with IgAN who currently have limited treatment options and high unmet need.”
IgAN is the most common primary glomerular disease globally with US prevalence of 140,000 to 150,000 patients.
Investment thesis
- Chinook merged with the struggling Aduro Biotech in October 2020 and has made steady clinical progress since then.
- The stock held relatively steady on Wednesday after the deal pricing was announced, trading around the offering price before closing at $13.59.
- We think that investors who can tolerate the binary event risks that are inherent in clinical-stage biotech have reasons to be optimistic about Chinook.
- The company's market capitalization of $860 million is modest given that it has an asset in Phase 3 trials for a disease with high unmet need.
- Chinook had $276 million in cash, equivalents and marketable securities as of March 31, 2022, which the company has said would provide runway "well into 2024."
- Investors should welcome the additional money raised in this deal to extend the runway even further.
- The FDA only has approved one steroidal treatment for IgAN.
- More than 50% of patients remain remain at risk for progression.
- Proteinuria is the most important predictor of kidney disease progression, and the company has consistently shown effective reduction of proteinuria in its trials.
- Proteinuria reduction is recognized by the FDA as a surrogate endpoint for accelerated approval (with full approval based on kidney function as measured by estimated glomerular filtration rate, or eGFR).
- In addition to the most recent data readouts, investors should expect numerous other clinical catalysts during 2022 and 2023.
- We like the combination of clinical progress and the well-stocked warchest.
[Note: Article was updated on May 26, 2022, to add a stock chart.]
_____
Source: Equities News



