Ardelyx Secures $27.5 Million Debt Financing From SLR Capital

Embattled biotech Ardelyx ARDX announced Thursday that it has entered into a debt financing agreement with SLR Capital Partners, a leading direct lender to middle market companies.
Under the terms of the agreement, SLR is providing a senior secured term loan facility, of which Ardelyx drew down $27.5 million at closing.
The loan has:
- An interest rate of 7.95% plus the 30-day LIBOR
- A maturity date of March 1, 2027
- Interest-only period through March 2024
Ardelyx will use the drawn funds to repay in full the 2018 term loan agreement that it has with Solar Capital and Western Alliance, under which the interest-only period had expired.
Ardelyx may borrow an additional $22.5 million from SLR on or before July 25, 2023, if:
- Ardelyx receives FDA approval for tenapanor for the control of serum phosphorus in adult patients with chronic kidney disease (CKD) on dialysis on or before December 31, 2022, and
- Ardelyx has achieved certain product revenue milestones targets.
FDA's surprise rejection of tenapanor in CKD in July 2021
Ardelyx gave the market an unwelcome surprise last summer when it announced that FDA found certain "deficiencies" in the company's Phase 3 trials of tenapanor in patients with CKD.
The FDA, in its complete response letter to Ardelyx, characterized the magnitude of the treatment effect of tenapanor in reducing serum phosphorus in CKD patients on dialysis as "small and of unclear clinical significance." Ouch.
Investment thesis
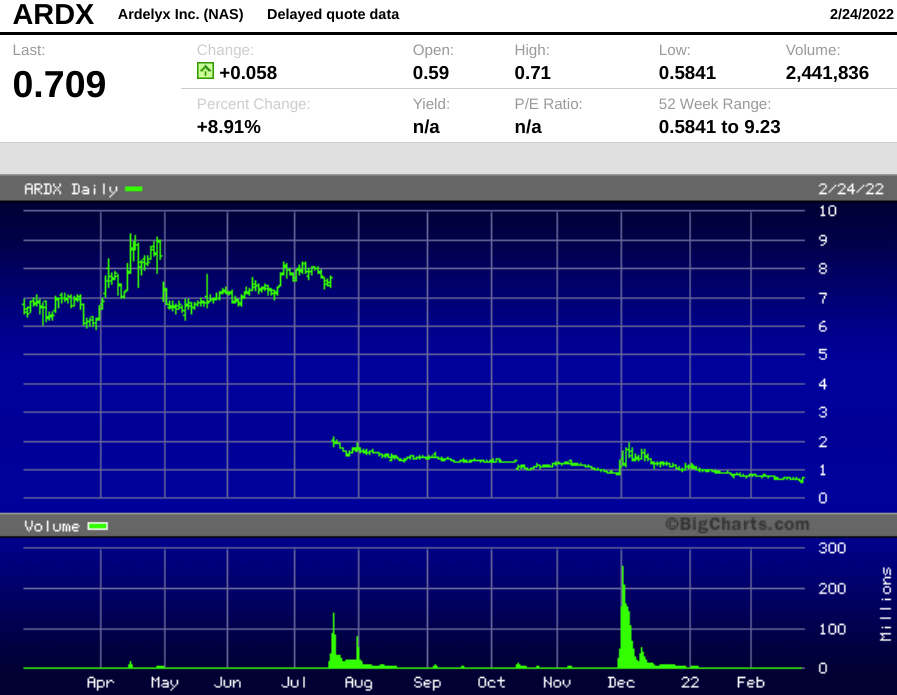
That rejection by the FDA erased 74% of Ardelyx's market value on July 20, 2021, and the stock has drifted even lower since then.
ARDX closed Thursday at $0.709 per share, up 8.9% on the day on the news of the new loan agreement. Even with the day's move, the company has a market cap of barely $80 million.
- The chart may be enough to scare away all but the most risk-tolerant investors, but at this level, the stock is effectively a call option on whether or not the FDA will approve tenapanor for CKD by the end of this year.
- Ardelyx has one FDA approval under its belt with tenapanor for irritable bowel syndrome with constipation (IBS-C) in adults.
- We don't think the market is giving the company much credit for the revenue potential of tenapanor in IBS-C, a condition that affects 11 million people in the US.
- The company had $142 million in cash, equivalents and short-term investments as of Sept. 30. 2021, which means the stock is trading for essentially pure cash value.
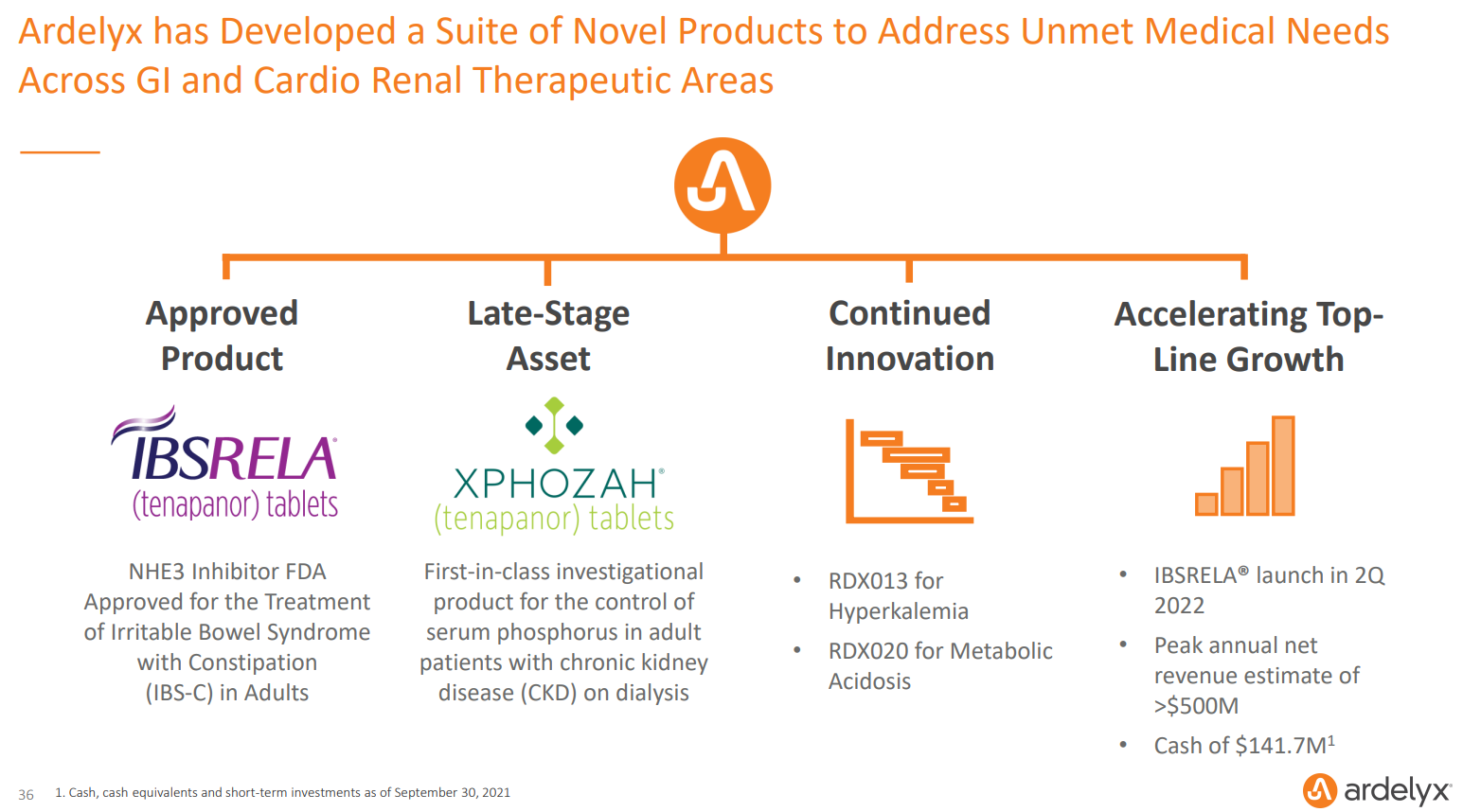 Image source: Ardelyx
Image source: Ardelyx
_____
Source: Equities News



