Affimed (AFMD) announced Tuesday that it had sold 22,500,000 common shares at $4.00 per share for gross proceeds of $90 million.
The Heidelberg, Germany-based company is developing therapeutics for a broad array of cancers.
Affimed had $224 million in cash and equivalents as of Dec. 31, 2021, which the company had stated would carry it through the second half of 2023.
The addition of about $84.6 million in net proceeds would add nearly another year of runway based on 2021's net cash outflow of $98 million.
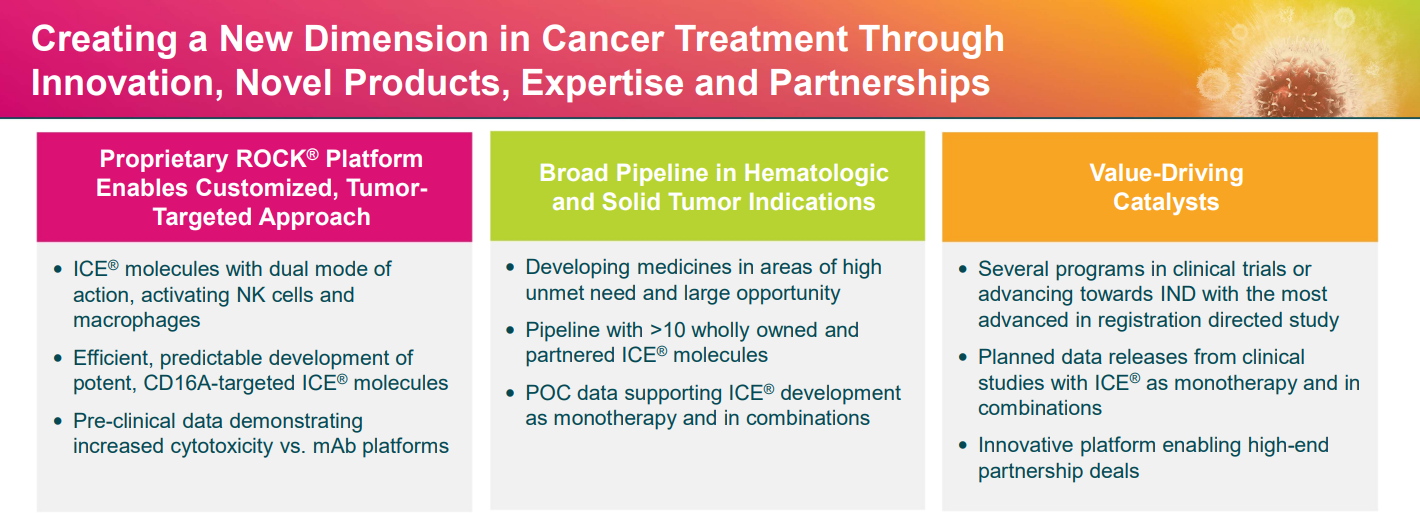
Image source: Affimed
Broad pipeline
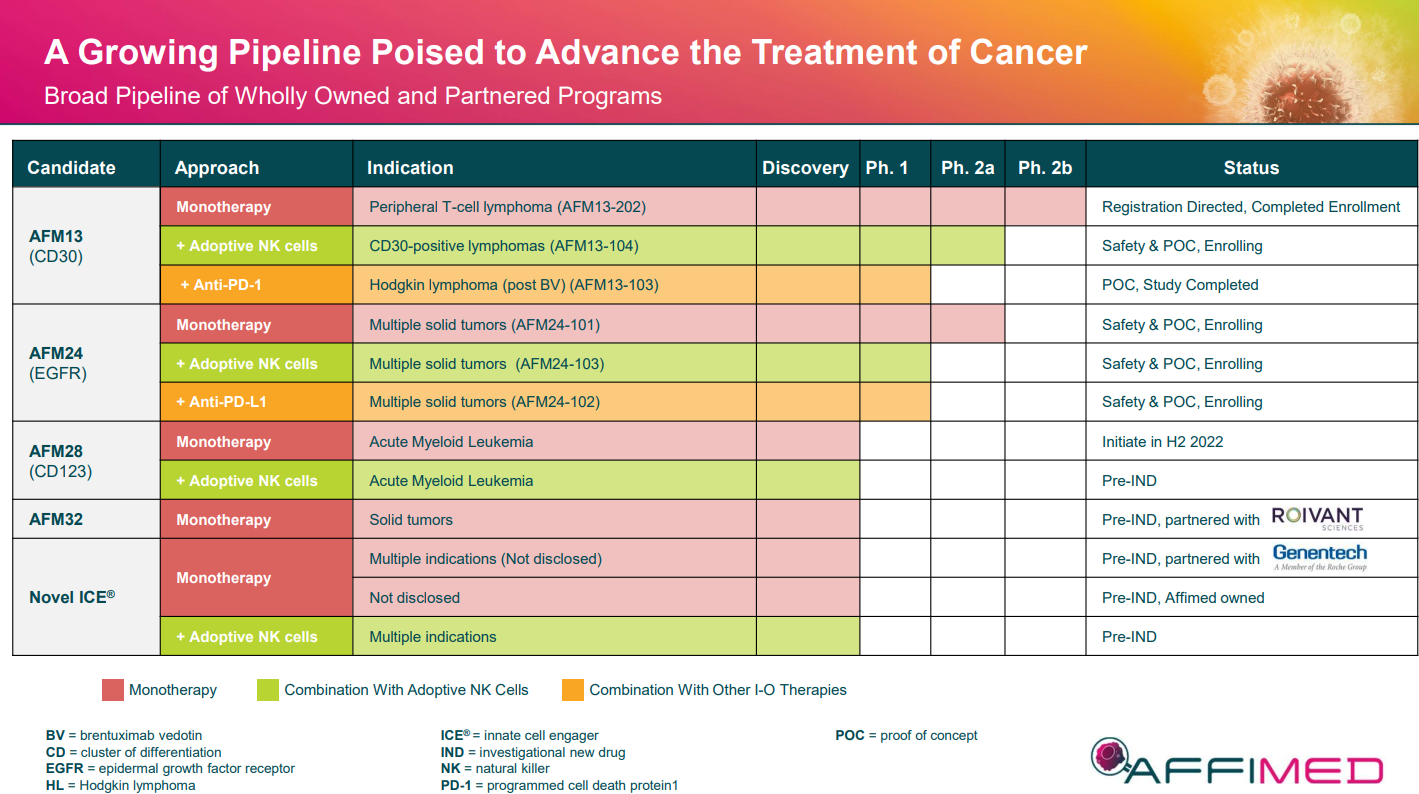
Affimed has developed what it calls innate cell engager (ICE) molecules designed to activate the innate immune system to provide targeted therapies for a variety of cancer indications.
The company's lead candidate, AFM13, is currently in a Phase 1/2 study at The University of Texas MD Anderson Cancer Center in patients with CD30-positive relapsed or refractory Hodgkin and non-Hodgkin lymphomas.
Affimed provided a data update last week at the American Association for Cancer Research (AACR) Annual Meeting 2022 that showed a 100% objective response rate (ORR) and an improvement of complete response (CR) rate to 62% at the recommended phase 2 dose in 13 patients after two cycles of therapy.
Investment thesis
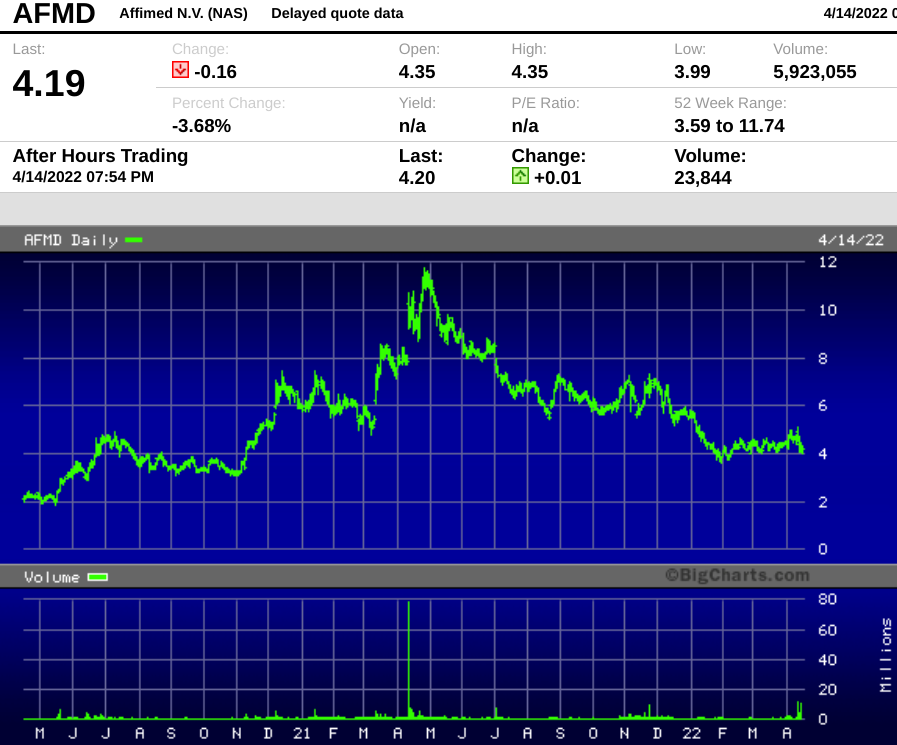
Affimed has been public since 2014. Long-term investors have had a couple of sharp peaks separated by deep valleys, and the stock has been a disappointment for much of the past year, losing 64% of its value since April 2021.
This isn't a quick trade by any means, but we think investors with at least a one-year time horizon and high risk tolerance have numerous reasons to be optimistic.
- Company has a broad pipeline targeting areas of high unmet need.
- Recent strong clinical data in CD30-positive lymphomas.
-
Multiple clinical catalysts in 2022:
-
AFM13
- Monotherapy in peripheral T-cell lymphoma (PTCL): Enrollment completed in January 2022; topline data expected in second half 2022
- NK cell combination in CD30+ lymphoma: Data updated at AACR 2022; additional updates and guidance on further development planned in second half 2022
-
AFM24
- Monotherapy: Determined recommended phase 2 dose, expansion cohorts enrolling, update at AACR and additional updates planned in second half 2022
- NK cell combination: Study initiated with updates expected in second half 2022
- Anti–PD-L1 checkpoint inhibitor combination: Study initiated with updates expected in second half 2022
-
AFM28
- Initial preclinical data presented at ASH 2021; Further data updates planned in Q2 and second half 2022
- IND filing with FDA expected in Q2 2022; Initiation of first-in-human clinical study expected in second half 2022
-
AFM13
- Clinical partnerships with companies such as Genentech, Merck and Roche and with institutions such as MD Anderson Cancer Center.
-
This latest equity raise puts cash and equivalents at over $300 million on a pro forma basis as of Dec. 31, 2021.
- Cash runway into second half of 2024.
_____
Source: Equities News



